This article focuses on the corrosion control techniques to be applied in hydrocarbon facilities and its implementation by proper materials selection and requirements for new project installations. This includes flow lines, production lines inside process facilities including all associated utility pipelines. The primary objective for establishing appropriate corrosion control strategies and materials selection is to ensure the safe and profitable utilization of the assets over the entire proposed life cycle.
Material Selection
The material selection shall be done by taking into consideration safe operation, total life cycle cost, reliability, fabrication, maintenance, weight, and availability of materials. All materials, equipment and corrosion management activities for a hydrocarbon facility shall be based on the specified design life. Carbon or low alloy steels are routinely used for hydrocarbon facilities (oil/gas production) provided that the rates of general and pitting corrosion are maintained within acceptable limits, either with or without the use of corrosion inhibitors. All corrosion allowances for carbon steel will be recommended to give an expectation of the equipment lasting for the design life. Preference shall be given to the use of corrosion resistant materials in place of carbon steel where the accumulated total life-time corrosion using carbon steel exceeds 3mm. It will provide improved plant reliability and availability, or it will reduce inspection and maintenance requirements. Industry practice and NACE allows the use of carbon steel with between 3mm (0.11811 inch) and 6mm (0.23622 inch) corrosion allowance. A critical assumption in the prediction of service life is that certain operations, such as maintenance, repair and chemical treatment activities will be performed to achieve the reliability. These activities include the operation of the facilities, corrosion inhibition, cathodic protection and internal and external coating maintenance.
Assessment of Corrosion
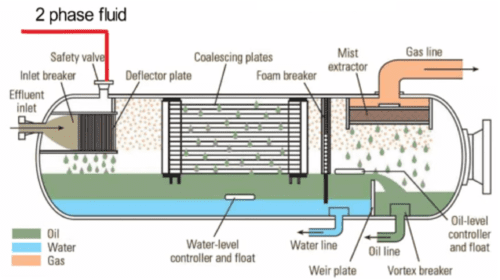
The corrosion rate predictions shall be used to estimate an uninhibited corrosion rate for carbon steel in the service environment and to determine the corrosion allowance that would be necessary for the design life. If this corrosion allowance is small enough to be economically and practically acceptable, carbon steel may be accepted, either with or without corrosion inhibition. If the required corrosion allowance without inhibition is excessive, the performance of corrosion inhibitor required to allow an acceptable corrosion allowance shall be calculated. The potential for corrosion in the hydrocarbon facility pipelines is largely dependent on the presence of “free” water, the presence of CO2 and H2S levels in the hydrocarbon, operating temperature and pressure, the amount of dissolved CO2 and H2S, the presence of dissolved oxygen, the flow regime, flow velocity and cavitation conditions, the presence of bacteria causing microbiologically influenced corrosion or MIC (Certain bacteria, generally those known by the collective term “Sulphate Reducing Bacteria” – SRB, cause MIC in hydrocarbon systems) and the presence and quantity of solids.
Condensing Phase Corrosion
Condensing phase corrosion can occur in wet hydrocarbon pipelines, where cooling of the hydrocarbon will result in condensation of water at the top of the pipe (i.e., top-of-line corrosion), under moisture condensing conditions in the vapor phase of plant piping, top-of-the-line corrosion can occur in long wet hydrocarbon and multiphase pipelines, where the corrosion inhibitor cannot remain suspended in the hydrocarbon phase, such as under stratified flow regimes. Top-of-line corrosion usually only becomes a problem if the condensation rate is high, the corrosivity is high due to high CO2 partial pressures and the temperature remains high at the point where inhibitor is totally lost from the hydrocarbon phase. Top-of-the-line corrosion rates in multiphase pipelines are usually lower than the bulk water phase at the bottom of the pipe because of the more limited exposure to the corrosive species and because the moisture condensation film is thin and becomes saturated with iron corrosion products, which stifles the corrosion reactions. Typically, a moisture condensation rate or less is applied for pipeline corrosion modeling within the vapor phase, which provides an order of magnitude reduction in corrosion rate compared with a bulk liquid phase. Once the pipeline fluids have reached temperature equilibrium with the external environment, the moisture condensation rate will normally be low, resulting in low corrosion rates.
Erosion
Erosion is the physical removal of wall material by the flowing process fluids. Erosion is a complex issue; dictated by fluid phase, flow regime, density, solids content, solids hardness and solids geometry. Erosion-corrosion and flow-enhanced corrosion occur when the corrosion product film is physically removed by high hydrodynamic shear stresses at the pipe wall or the impingement of solids or liquids on the pipe wall. The primary method of avoiding erosion and erosion-corrosion is to design facilities with velocities below the limit given by API RP 14E which provides a reliable design that is tolerant of design changes and unexpectedly erosive flows (e.g., from slugs of solids). The U.K. Health and Safety Executive Research Report 115 provide a useful additional guide for systems for which API RP 14E may not be applicable.
Galvanic Corrosion
Galvanic corrosion occurs where two dissimilar metals are bolted or welded together in the presence of a conductive, corrosive environment. In the presence of an electrolyte, differences in the electrochemical potentials of the metals can drive corrosion into the more electronegative material, resulting in localized corrosion around the metal join. The speed and magnitude of the corrosion depends principally on the electrochemical potential difference between the two metals, the resistance of the joint and the electrolyte, the electrochemical kinetics of the corrosion processes in the electrolyte, the geometry of the join (exposed cathode to anode area ratios and distances), the presence of coatings, the presence of corrosion inhibitor, which will reduce the corrosion rate of carbon steel and may prevent galvanic corrosion by forming a barrier film across the interface, thus preventing ionic flow through the bulk electrolyte between the two metals. Dissimilar metals should not be joined where possible, particularly in wet or corrosive environments. Insulating joints are not generally encouraged due to the difficulty in effecting electrical isolation and the potential for hidden corrosion when there is inadvertent short-circuiting or when the insulating sleeves fitted to the bolts trap moisture. There is also a possibility of generation of a spark should the joint be inadvertently shorted during maintenance or other activities. Insulating gasket kits to be used to control galvanic corrosion shall also be not allowed. Coatings shall be allowed to be used to control galvanic corrosion. It is more effective to coat the cathodic material than the anodic material, since this reduces the effective cathode-to-anode area ratio.
Under Deposit Corrosion
Under deposit corrosion occurs in wet liquid hydrocarbon systems where solids, usually sand from the reservoir or corrosion products, settle out in low flow or stagnant areas. The solids themselves can cause preferential corrosion under the deposit due to the differences between the chemical environment under the deposit and the bulk flow. They can also prevent corrosion inhibitor reaching the steel beneath the deposit and can disrupt the formation of protective corrosion product films, or provide a breeding ground for corrosion inducing bacteria (e.g., MIC). As per good design practice, all systems should be designed to be free-flowing, avoiding dead legs, low spots and vertical routings, and should have sufficient velocity that water and solids remain suspended in the flow, and cannot become trapped in low spots in the system. Where solids may be expected to settle and cannot be removed upstream, carbon and stainless-steel pipelines should be provided with pipeline scraping / pigging facilities to permit cleaning and mechanical removal of deposits.
Corrosion Monitoring
Corrosion monitoring is to ensure that the design life will not be adversely compromised during service and to ensure the safe and economic operating life of a facility. The monitoring may also be used to optimize inspection intervals that will invalidate inspection periods or endanger the plant. In particular, corrosion monitoring is required where corrosion is controlled by chemical injection (corrosion inhibitor, oxygen scavenger, or pH stabilization). Other factors which may be considered in determining a requirement for corrosion monitoring include changes in the operating environment which can lead to a significant increase in the corrosivity of the environment towards carbon steel, either with or without corrosion inhibitors, on pipelines with zero corrosion allowance. The outcome of corrosion monitoring can lead to timely re-assessment and adjustment of the system of corrosion management and also could lead to optimization of operational costs (inhibitor dosing, inspections, cleaning, etc.). The functional requirement of the corrosion monitoring system is that it shall detect and quantify trends in the corrosivity of the fluids and shall do so within a time frame short enough to enable corrosion mitigation measures to be instigated or adjusted before significant metal loss has occurred.
Corrosion Control Implementation
If aqueous phase water is absent, zero corrosion rates shall be assumed and carbon steel can be used. If aqueous phase water is present, apply de Waard and Lotz 93 to calculate the corrosion rate of carbon steel in the absence of corrosion inhibitor. If the aqueous phase water is only present because of condensation, consider the applicability of the moisture condensation rate criteria for applying an order of magnitude reduction in corrosion rate; determine the accumulated corrosion of carbon steel over the design life in the absence of corrosion inhibitor from the uninhibited corrosion rate calculated. If the accumulated corrosion of carbon steel over the design life is ≤ 3mm, adopt carbon steel with the appropriate corrosion allowance as the material of construction without the use of corrosion inhibitor. If the accumulated corrosion of carbon steel over the design life is > 3mm, determine whether corrosion inhibitor at appropriate availabilities or efficiencies is able to reduce it to ≤ 3mm, adopt carbon steel with the appropriate corrosion allowance as the material of construction and apply corrosion inhibitor. If the accumulated corrosion is > 3mm, consider the use of carbon steel with up to 6mm corrosion allowance. Where carbon steel cannot be used, choose a suitable corrosion-resistant alloy (CRA).
Nirmal Surendran Menon, PMP®, is the author of the book, Valves Made Easy, along with a coauthor, who is an industry expert as well as a project management professional with 17-plus years of experience in the oil and gas/energy facility construction. He currently works as a senior construction manager overseeing the Pipe Pressure Testing and Pipe Cleaning portfolio with an EPC company with a distinguished reputation in the industry. Menon’s interests include pipe integrity, pipe cleanliness, loss prevention, and human factors for process facilities. He has authored 11 articles and two books, and has sustained national and international acclaim in the industry, having been part of major LNG project construction execution in different geographical locations.
Oil and gas operations are commonly found in remote locations far from company headquarters. Now, it's possible to monitor pump operations, collate and analyze seismic data, and track employees around the world from almost anywhere. Whether employees are in the office or in the field, the internet and related applications enable a greater multidirectional flow of information – and control – than ever before.